Bcs Class 2 Drugs List
This is the list of Schedule II drugs as defined by the United StatesControlled Substances Act.[1]The following findings are required for drugs to be placed in this schedule:[2]
BCS Class 1 and 3 drugs for Biowaiver. • Updates on. Class 2: Low Solubility / Highly Permeable (LS/HP). Drug substances selected based on WHO's List of.
- The drug or other substance has a high potential for abuse.
- The drug or other substance has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions.
- Abuse of the drug or other substances may lead to severe psychological or physical dependence.
The complete list of Schedule II drugs follows.[1] The Administrative Controlled Substances Code Number for each drug is included.
| ACSCN | Class | Drug |
|---|---|---|
| 9050 | opiate | Codeine |
| 9334 | opiate | Dihydroetorphine |
| 9190 | opiate | Ethylmorphine |
| 9059 | opiate | Etorphine hydrochloride |
| 9640 | opiate | Granulated opium |
| 9193 | opiate | Hydrocodone |
| 9150 | opiate | Hydromorphone |
| 9260 | opiate | Metopon |
| 9300 | opiate | Morphine |
| 9610 | opiate | Opium extracts |
| 9620 | opiate | Opium fluid |
| 9330 | opiate | Oripavine |
| 9143 | opiate | Oxycodone |
| 9652 | opiate | Oxymorphone |
| 9639 | opiate | Powdered opium |
| 9600 | opiate | Raw opium |
| 9333 | opiate | Thebaine |
| 9630 | opiate | Tincture of opium |
| opiate | Opium poppy and poppy straw | |
| 9040 | stimulant | Coca, leaves and any salt, compound, derivative or preparation of coca leaves |
| 9041 | stimulant | Cocaine, and its salts, isomers, derivatives and salts of isomers and derivatives |
| 9180 | stimulant | Ecgonine, and its salts, isomers, derivatives and salts of isomers and derivatives |
| 9670 | opiate | Concentrate of poppy straw (the crude extract of poppy straw in either liquid, solid or powder form which contains the phenanthrene alkaloids of the opium poppy) |
| 9737 | opioid | Alfentanil |
| 9010 | opiate | Alphaprodine |
| 9020 | opioid | Anileridine |
| 9800 | opiate | Bezitramide |
| 9273 | opioid | Bulk dextropropoxyphene (non-dosage forms) |
| 9743 | opioid | Carfentanil |
| 9120 | opiate | Dihydrocodeine |
| 9170 | opioid | Diphenoxylate |
| 9801 | opioid | Fentanyl |
| 9226 | opioid | Isomethadone |
| 9648 | opiate | Levo-alphacetylmethadol |
| 9210 | opiate | Levomethorphan |
| 9220 | opiate | Levorphanol |
| 9240 | opioid | Metazocine |
| 9250 | opioid | Methadone |
| 9254 | opiate intermediate | Methadone intermediate: 4-cyano-2-dimethylamino-4,4-diphenyl butane |
| 9802 | opiate intermediate | Moramide intermediate: 2-methyl-3-morpholino-1,1-diphenylpropane-carboxylic acid |
| 9230 | opioid | Pethidine (meperidine) |
| 9232 | opiate intermediate | Pethidine intermediate A: 4-cyano-1-methyl-4-phenylpiperidine |
| 9233 | opiate intermediate | Pethidine intermediate B, ethyl-4-phenylpiperidine-4-carboxylate |
| 9234 | opiate intermediate | Pethidine intermediate C, 1-methyl-4-phenylpiperidine-4-carboxylic acid |
| 9715 | opiate | Phenazocine |
| 9730 | opiate | Piminodine |
| 9732 | opiate | Racemethorphan |
| 9733 | opiate | Racemorphan |
| 9739 | opiate | Remifentanil |
| 9740 | opiate | Sufentanil |
| 9780 | opiate | Tapentadol |
| 1100 | stimulant | Amphetamine, its salts, optical isomers, and salts of its optical isomers (Adderall) |
| 1105 | stimulant | Methamphetamine, its salts, isomers, and salts of its isomers |
| 1631 | stimulant | Phenmetrazine and its salts |
| 1724 | stimulant | Methylphenidate (Ritalin, Concerta, etc.) |
| 1205 | stimulant | Lisdexamfetamine (Vyvanse), its salts, isomers, and salts of its isomers |
| 2125 | depressant | Amobarbital |
| 2550 | depressant | Glutethimide |
| 2270 | depressant | Pentobarbital |
| 7471 | depressant | Phencyclidine |
| 2315 | depressant | Secobarbital |
| 7379 | hallucinogen | Nabilone |
| 8501 | precursor | Phenylacetone |
| 7460 | precursor | 1-phenylcyclohexylamine |
| 8603 | precursor | 1-piperidinocyclohexanecarbonitrile (PCC) |
| 8333 | precursor | 4-anilino-N-phenethyl-4-piperidine (ANPP) |
References[edit]
- ^ ab21 CFR1308.12 (CSA Sched II) with changes through 77 FR64032 (Oct 18, 2012). Retrieved September 6, 2013.
- ^21 U.S.C.§ 812(b)(4) retrieved October 7, 2007
The Biopharmaceutics Classification System is a system to differentiate the drugs on the basis of their solubility and permeability.[1]
This system restricts the prediction using the parameters solubility and intestinal permeability. The solubility classification is based on a United States Pharmacopoeia (USP) aperture. The intestinal permeability classification is based on a comparison to the intravenous injection. All those factors are highly important because 85% of the most sold drugs in the United States and Europe are orally administered[citation needed].
BCS classes[edit]
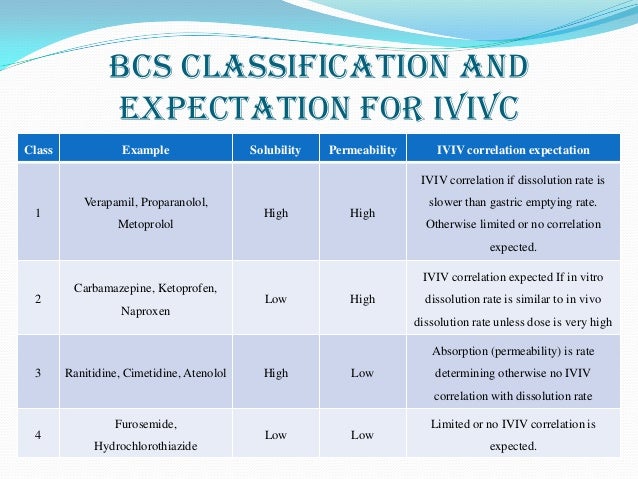
According to the Biopharmaceutical Classification System (BCS) drug substances are classified to four classes upon their solubility and permeability:[1]
- Class I - high permeability, high solubility
- Example: metoprolol, paracetamol[2]
- Those compounds are well absorbed and their absorption rate is usually higher than excretion.
- Class II - high permeability, low solubility
- Example: glibenclamide, bicalutamide, ezetimibe, aceclofenac
- The bioavailability of those products is limited by their solvation rate. A correlation between the in vivo bioavailability and the in vitro solvation can be found.
- Class III - low permeability, high solubility
- Example: cimetidine
- The absorption is limited by the permeation rate but the drug is solvated very fast. If the formulation does not change the permeability or gastro-intestinal duration time, then class I criteria can be applied.
- Class IV - low permeability, low solubility
- Example: Bifonazole
- Those compounds have a poor bioavailability. Usually they are not well absorbed over the intestinal mucosa and a high variability is expected.
Definitions[edit]
The drugs are classified in BCS on the basis of solubility, permeability, and dissolution.
Solubility class boundaries are based on the highest dose strength of an immediate release product. A drug is considered highly soluble when the highest dose strength is soluble in 250 ml or less of aqueous media over the pH range of 1 to 7.5. The volume estimate of 250 ml is derived from typical bioequivalence study protocols that prescribe administration of a drug product to fasting human volunteers with a glass of water.
Permeability class boundaries are based indirectly on the extent of absorption of a drug substance in humans and directly on the measurement of rates of mass transfer across human intestinal membrane. Alternatively non-human systems capable of predicting drug absorption in humans can be used (such as in-vitro culture methods). A drug substance is considered highly permeable when the extent of absorption in humans is determined to be 90% or more of the administered dose based on a mass-balance determination or in comparison to an intravenous dose.
For dissolution class boundaries, an immediate release product is considered rapidly dissolving when no less than 85% of the labeled amount of the drug substance dissolves within 15 minutes using USP Dissolution Apparatus 1 at 100 RPM or Apparatus 2 at 50 RPM in a volume of 900 ml or less in the following media: 0.1 N HCl or simulated gastric fluid or pH 4.5 buffer and pH 6.8 buffer or simulated intestinal fluid.
Bcs Class 2 Drugs List Pdf
See also[edit]
- ADME
References[edit]
- ^ abMehta M (2016). Biopharmaceutics Classification System (BCS): Development, Implementation, and Growth. Wiley. ISBN978-1-118-47661-1.
- ^https://www.ema.europa.eu/documents/scientific-guideline/draft-paracetamol-oral-use-immediate-release-formulations-product-specific-bioequivalence-guidance_en.pdf
Bcs Class 3 Drugs List Pdf
Further reading[edit]
- Folkers G, van de Waterbeemd H, Lennernäs H, Artursson P, Mannhold R, Kubinyi H (2003). Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability (Methods and Principles in Medicinal Chemistry). Weinheim: Wiley-VCH. ISBN3-527-30438-X.
- Amidon GL, Lennernäs H, Shah VP, Crison JR (March 1995). 'A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability'. Pharm. Res. 12 (3): 413–20. PMID7617530.
Bcs Class 2 Drugs List
External links[edit]
- BCS guidance of the U.S. Food and Drug Administration



